Home » De Simone Formulation®
De Simone Formulation®

De Simone Formulation®
(Available as Visbiome in USA) and Vivomixx in Europe)
Powered by the proprietary De Simone Formulation, DSF is a high potency probiotic medical food containing 8 strains of live bacteria in concentrations of 112.5, 225 billion CFU per capsule and 450 and 900 billion CFU per sachet – one of the highest concentrations available.
With the exclusive patented Di Simone Formulation, which is backed by more than 70 published human clinical studies your product gets a marketing leverage unlike others.
The De Simone Formulation is a probiotic medical food containing 8 strains of living bacteria. It is the driving force behind De Simone Formulation, which aids in the dietary management of microbial imbalance associated with stomach and intestinal disorders like IBS, ulcerative colitis, pouchitis, and hepatic encephalopathy.
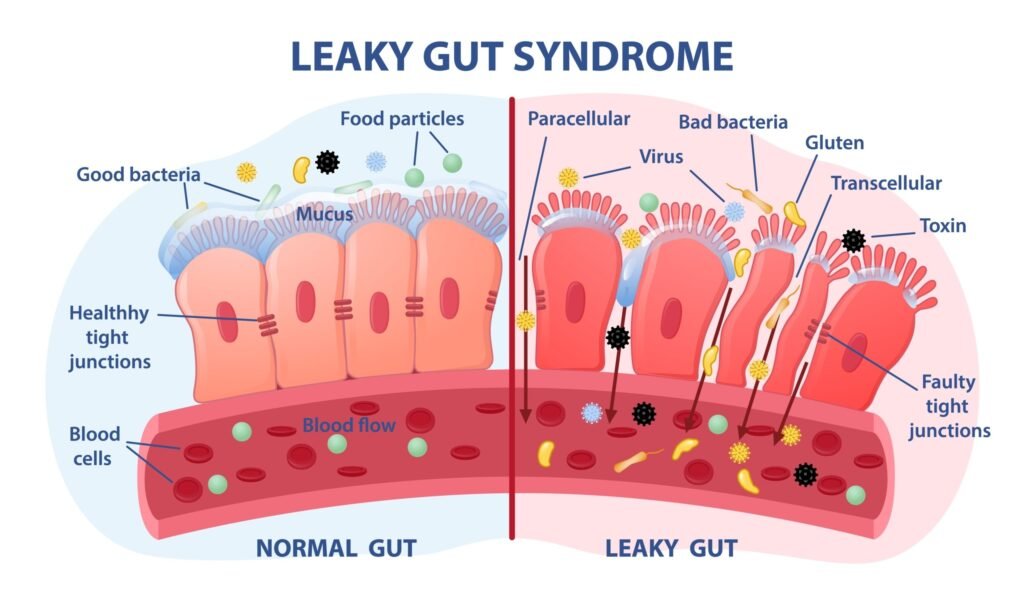
Leaky Gut Syndrome and De Simone Formula® (DSF®)
Leaky gut syndrome and intestinal barrier dysfunction are associated with intestinal diseases, such as Inflammatory Bowel Disease and Irritable Bowel Syndrome, as well as extra-intestinal diseases, including heart diseases, obesity, type 1 and type 2 diabetes mellitus, and neurological diseases.
Given the relationship between intestinal permeability and numerous metabolic diseases, it is an excellent strategy to avoid or minimize intestinal permeability.
Our proprietary De Simone Formulation (DSF) – a unique blend of 8 carefully selected and extensively tested probiotics, together with good diet and lifestyle, is an intelligent approach to restore gut microbiome, maintain gut lining integrity and avoid leaky gut, thus minimizing various metabolic syndrome conditions.
8
Strains of Live Bacteria
20+
Years of Research
100+
Peer-Reviewed Clinical Studies
Key attributes of the De Simone Formulation® include:
Highly concentrated natural yogurt bacteria which are grown using dairy ingredients to ensure their health and vitality.
The bacterial strains have been selected due to their individual characteristics and synergistic activities. They are cultured from proprietary Master Cell Banks and manufactured by Dupont USA, and then blended using specific ratios as per the work done by Professor De Simone.
Lactobacillus paracasei
DSM 24733/SD5218
Lactobacillus plantarum
DSM 24730/SD5209
Lactobacillus acidophilus
DSM 24735/SD5212
Lactobacillus delbrueckii subspecies bulgaricus*
DSM 24734/SD5210
Bifidobacterium longum ±
DSM 24736/SD5220
Bifidobacterium infantis
DSM 24737/SD5219
Bifidobacterium breve
DSM 24732/SD5206
Streptococcus thermophiles
DSM 24731/SD5207
More about Professor De Simone

Dosing
| Product | Bacteria Count (in CFUs) | Format/Flavor |
|---|---|---|
| DSF/Visbiome/Vivomixx Capsules | 112.5 Billion per serving | Capsule available in pack of 10/30 |
| DSF/Visbiome/Vivomixx Capsules | 225 Billion per serving | Capsule available in pack of 10/30 |
| DSF/Visbiome/Vivomixx Unflavored Powder | 450 Billion per packet | Sachet available in pack of 10/ 30 per box |
| DSF/Visbiome/Vivomixx Extra Strength (Dispensed By Prescription) | 900 Billion per packet | Sachet available in pack of 10/ 30 per box |
Disease specific dosing recommendations for adults
| For the Dietary Management of | Visbiome Packets Per Day (450 Billion) | Capsules Per Day | Visbiome Extra Strength Packets Per Day (900 Billion) |
|---|---|---|---|
| Irritable Bowel Syndrome (IBS) | ½-1 | 2-4 | ¼-½ |
| Antibiotic Associated Diarrhea (AAD) Under Antibiotic Treatment | 1 | 4 | n/a |
| Antibiotic Associated Diarrhea (AAD) After Antibiotic Treatment | ½ | 2 | n/a |
| Ulcerative Colitis (maintenance) | 1-2 | 4-8 | ½-1 |
| Active Ulcerative Colitis (flaring) | 4-8 | NA | 2-4 |
| Pouchitis | 2-4 | NA | 1-2 |
| Hepatic Encephalopathy (HE) | 1-2 | 4-8 | ½-1 |
Disease specific dosing recommendation for children / infants
| For the Dietary Management of | Less Than 2 Years | 2-5 Years | 6-11 Years | 12-17 Years |
|---|---|---|---|---|
| Irritable Bowel Syndrome (IBS) | ¼ Packet | ½ Packet | ½ Packet | ½-1 Packet |
| Ulcerative Colitis (maintenance) | ¼ Packet | ½ Packet | ½-1 Packet | 1-2 Packets |
| Active Ulcerative Colitis (flaring) | ¼ - ½ Packet | ½-1 Packet | 1-2 Packets | 2-4 Packets |
Possible adverse effects
Mild abdominal bloating has been occasionally reported during the first few days of consuming DSF. This is generally a readjustment of the microbiota, which usually diminishes within 3-4 days. If bloating persists, the patient should consult their physician and consider reduce their intake of product for a few days


Drug Interactions
There are no known adverse drug interactions associated with the consumption of VISBIOME. The bacteria in VISBIOME may be inactivated by certain antibiotics. When taking an antibiotic with VISBIOME, consume the antibiotic four hours prior or after VISBIOME consumption.
Contraindications / Warnings and Precautions
Contraindicated for premature infants in the NICU setting
Pregnant women and lactating mothers are advised to use this product only after doctor’s consent and advice

Dairy, GMO, Gluten, And Kosher Status
Clinically Proven to be Effective
DSF® has been the subject of over 100 peer-reviewed trials making it the most studied multi-strain probiotics available.

Low FODMAP Certified
Monash University, the leader in FODMAP research, has officially certified DSF® as a Low FODMAP food.

Gluten-Free
DSF® is Gluten-Free, Non-GMO, Cornstarch-Free, and Vegetarian.

Lactose - Free
Lactose was not detectable in amounts greater than 50 parts per million (PPM). Trace amounts of milk may be present in the final product.

High Potency Protected
DSF® is shipped and stored under refrigerated conditions (36-46°F, 2-8°C) with a temperature card to ensure maximum potency.

Manufactured in the US
DSF® is proudly manufactured in the USA, it is kosher certified and Halal suitable.